Identifying bees that can manage their own varroa populations
The initial phase of the project used biotechnical methods (eg; queen frame trapping) to replace chemical treatments. These are very effective at reducing varroa loads ahead of the winter bees being laid (see graph above) and winter colony survival is excellent. It felt an important step to overcome the pervasive fear in beekeeping that: “If you don’t treat your bees for varroa, they will die”. The bees certainly seemed fresher in the spring and research from Dr Ralph Buchler demonstrated that spring colonies were significantly larger using queen frame traps rather than treating (ref: National Honey Show 2019).
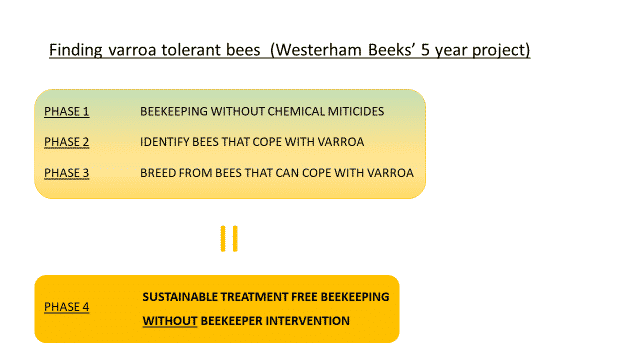
The project, which was planned in 2017 after meeting Dr Ralph Buchler at Gormanston that year, started for the 2018 season with a group of like-minded beekeepers. No miticide treatments were employed after 2017.
We mapped out the project without knowing the time frames between different phases and tentatively estimated a 5 year time frame.
Identifying varroa resistant traits in honeybees
The second phase of the project focused on identifying the characteristics of bees where colonies already manged their own varroa populations. What actions were the bees taking? What could we learn from them?
Thankfully, these are questions that many of the world’s leading bee scientists are focused on. There are adapted and unmanaged (for varroa) stocks of Apis mellifera (our western honeybee) in the UK, Continental Europe and the Americas. Barbara Locke summarised many of these in her research paper “Natural Varroa mite-surviving Apis mellifera honeybee populations”
https://link.springer.com/article/10.1007/s13592-015-0412-8
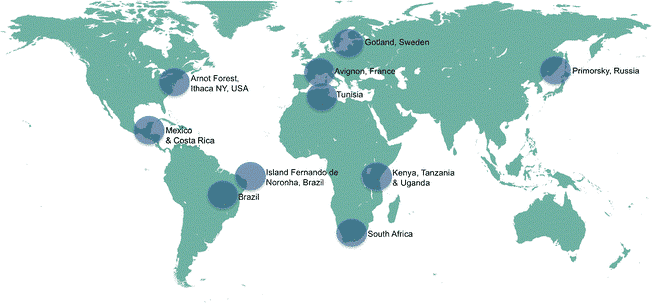
Working from bee scientist research on how adapted bees had become resistant to varroa, and together with our own observations, we formulated a proxy for beekeepers to identify bees that were managing their own mite populations.
Monitoring for Varroa resistant traits
In our colonies, we decided to monitor for the traits of naturally resistant bees, which bee scientists were unearthing across the UK, mainland Europe and the Arnot Forest. There are some free-living (unmanaged) colonies in the area, but insufficient to be a large influence over the gene pool. Together with our own data, we focus on:-
- Observing uncapping/recapping during colony inspections
- Monitoring chewed out infected pupae exoskeleton on insert boards, where the reproduction of Varroa was being interrupted
which produce……..
3. Low mite (drop) numbers & Deformed wing virus (“DWV”).

The graph below illustrates the high level of uncapping and recapping in “Mite Resistant” colonies versus “Mite Susceptible” colonies (referred to in this research as “Surviving” or “Susceptible”). Who would have thought all this uncapping/recapping activity was going unnoticed and not understood?
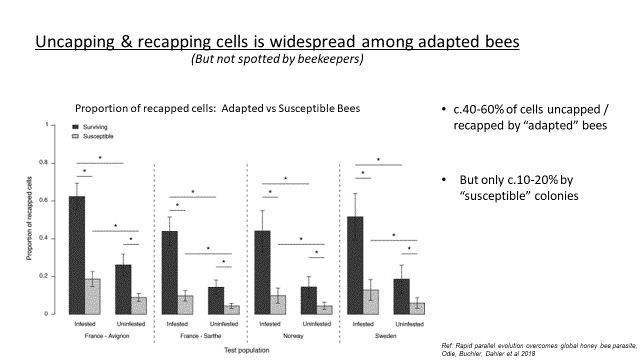
Recapped cells are harder to identify by the bee keeper; often they are slightly “domed” and sometimes a change in colour is noticeable where the “bee made” hole in the cell has been repaired.
Monitoring using an insert board for mites and hygienic behaviour
Mite numbers on an insert board are a broad quantitative indicator from 100% of the colony, as is counting mites in a 300 bee sample (from under 1% of the colony). We like the insert board as it is non-invasive, useable from January to December and provides a wealth of information on the colony’s activities, including hygienic behaviour against Varroa. The picture below shows parts of white pupae exoskeleton which has been chewed out by the bees. This can be seen from January onwards, when over wintering Varroa try to reproduce in the first of the season’s brood. Already, the bees are reducing mite growth and limiting DWV in the colony. Monitoring mite drop, which is carried out at the same time, corroborates the success of the bees’ strategy (or not).

Collecting mite data also helps to inform decisions when selecting queens. In the Spring, strong brood development provides many opportunities for Varroa to reproduce. Without interruption by the bees, mite numbers multiply rapidly in “Susceptible” colonies. The chart below shows the accumulated mite drop from late winter into the Spring from hives in the same apiary and all on National brood boxes.

In about 3 months for hive 8, over 2,000 mites had dropped versus under 500 from the other colonies. In the old regime, treatments would have kept these weak traits circulating – the opposite to good husbandry. This colony was moved out of the area. Another option early in the season is to requeen (we stopped treating in 2017 and culling doesn’t come naturally to beekeepers). Key to rapidly increasing the proportion of resistant traits in your locality is to remove the Varroa susceptible genetics.
Interpretation of mite drop & hygienic behaviour
We found that correlating seasonal changes in the colony with the natural mite drop enhanced our understanding and interpretation.
This next chart is intended as a reference point for mite drop & hygienic behaviour through a whole season (2021) in a resistant colony, where there is no beekeeper reduction of Varroa. It maps the seasonal brood availability during the spring build up and summer flow, where mites have most opportunities to reproduce, together with uncapping (“Un”) and pupae exoskeleton on the insert board (“P”). For colony size reference, it is in an insulated National brood box with queen excluder and had 5 supers through the season.

Underlying mite population (indicated by mite drop)
A resistant colony will start and end the year with roughly the same number of mites. The underlying mite population will grow in the Spring with the strong development of worker brood, boosted by the availability of drones from late March onwards. Mite levels then fall during a broodless period after an artificial swarm. When the new queen starts laying, mites move back into the brood so expect mite drop to fall. Interestingly, at this time during a strong summer nectar flow, we see a reduction of hygienic behaviour as the colony focuses on processing large amounts of nectar, prioritising the stores it requires for winter. This leads to reproductive success for the mites and growth in their numbers through this period. Warning: this won’t be seen in the mite drop as the Varroa are in the brood and can lead to a misinterpretation for the “once-a-year” mite counter or 300 bee sampler.
At the end of the summer nectar flow comes a “mass mortality” clean out of mites. We see this in every colony and the spike in mite-drop can be alarming when first experienced. In the colony shown, just over 50% of the mite drop for the full year occurred in four weeks. The bees were naturally achieving a reduction in Varroa akin to the application of a miticide treatment over the period. With this mite decrease goes a commensurate reduction in DWV load in the colony – very important for winter survival.
What’s behind this spike?
We believe there are a combination of factors:-
- The trigger is a reduction in worker brood as the queen reduces her laying rate into the autumn.
- This leads to more mites on the bees rather than safely reproducing in the cells. As we saw during the broodless period following the artificial swarm, mites will fall off.
- With less cells to breed in, multiple foundress mites enter the same cells to attempt reproduction. This is not good news for the predated-on larvae/pupae, but also leads to a high number of unsuccessful mite matings. On the insert board, we see c.25% juvenile mites in addition to the dark brick-red foundress mites. Whole families of mites are dying.
- The bees move into pre-winter behavioural mode. As well as the increased use of propolis around the brood nest, hygienic activity against varroa resumes following the collection of sufficient winter stores. Torben Schiffer describes this behavioural shift as “Stores Secure” (Beekeeping (R)evolution). For this reason, a full super of honey is left on the colonies. Note: During this period, beekeepers seeking to maximise their honey yield and replacing the bees’ winter stores with sugar water will change the bees’ priorities away from hygienic behaviour.
Spreading resistant traits
At Westerham Beekeepers, there are around 100 colonies not using chemical miticides. We are in build-out mode, looking to spread the best of the resistant genetics around the area from (artificial) swarms, sharing queens and from drones. The latter are a clone of the queen’s genetics, so we see them as a local resource by adding an empty brood frame on the outside of the brood nest. The frame in the picture hatched out over 3,000 drones – the modern day “Influencers”.

“Breeding” protocol
Not so much breeding, but identifying traits of varroa resistance from bees that have already demonstrated uncapping and low mite loads. We also like to see good bee health, evidenced by strong foraging and brood development in the spring, plus gentle temperament.
A key test is to over-winter the colonies without any beekeeper reduction of varroa. Importantly, this introduces an element of natural selection, which stops the cycle of breeding from bees that are susceptible to mites – that’s what we have been doing for some 30 years since varroa arrived in the UK. There is an element of judgement here for the beekeeper looking at uncapping and mite loads. A cautious approach is sensible, starting with just some of your colonies. It is also worth over-wintering some nucs as back-up.

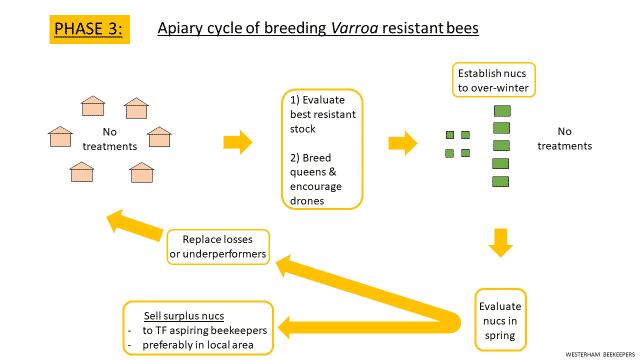
The slide below illustrates an apiary set-up to evaluate and breed varroa resistant bees, including nuc back-ups to produce surplus bees for any losses, replace underperformers or to sell.
Colony outcomes
We often get asked about colony losses, which we monitor for the starter group from 2017. Overwintered colonies number around 40 (±5) and the annual survivor-loss ratio to 2022 was 86%-14%, which compared to 82%-18% in the South East from BBKA surveys. There were a range of experiences, with one apiary recording no losses over that period. This is where working as a group pays off, as losses from Varroa are being compensated with better shared genetics from the other beekeepers.
Mistakes
We made a few…… Judgements are being made in a new area of beekeeping without mentors or mainstream education. Expect to make some errors. Here are a few of ours:-
- Falling in love with a well-behaved colony that had a high-ish mite load, especially when spare queen cells became available. Rarely ends well.
- Mite mutilation / grooming not being a key trait of resistance. Wasted many hours peering down a low powered microscope and cheering at missing body parts of Varroa and damaged carapaces. Couldn’t understand at the time why a “mutilator” colony harboured a very high mite load.
- Under appreciating that it could take 3+ years for resistant genetics to embed, after being over optimistic after early year successes.
- We trialled small cell foundation throughout two apiaries. Mite loads were not noticeably lower across the board, but only where colonies had sufficient resistant traits. We concluded there was no correlation between the two.
Getting started at a club
These are a summary of ideas based around how our group came together at Westerham Beekeepers.
- Form a group of like-minded beekeepers & set up a WhatsApp group to share experiences
- Look for the key traits of uncapping/recapping, chewed out pupae exoskeleton & low mite numbers
- Keep good colony records and embed your monitoring practices into hive record cards
- Start cautiously as there has been 30+ years of not selecting for varroa resistance
- Share the best genetics between beekeepers and help out with losses if someone has poor experience
- Raise your own queens and locally adapted bees – control the spread of the best genetics around your apiaries, including drone production
Westerham Beekeepers have also been supporting new initiatives at other beekeeping clubs including apiary workshops and hope to short-cut their learnings from ours. In due course, it would be good for the understanding of the key traits of Varroa resistance and selection processes to be incorporated into mainstream beekeeper education.
Looking for Varroa resistance traits re-engages the beekeeper in the interaction between your honey bees and the mites. It is fascinating to observe a host / parasite equilibrium developing as the honeybees interrupt the Varroa reproduction process and limit their impact on the colony. Breaking the cycle where regular miticide treatments lead to weak genetics, whilst increasing Varroa resistance traits is important for the long-term sustainability of the honey bee. We wish you well in your endeavours and let us know if we can help. https://westerham.kbka.org.uk/contact-us/
Acknowledgements
We are grateful for the mentorship of Dr Ralph Buchler in helping to initiate this project. In more recent years, Professor Stephen Martin (University of Salford) has made a significant contribution to our understanding around the areas of recapping and DWV. Thank you also to Tom Seeley for his insights and encouragement.